The gut-brain axis is a well established connection suggesting that the intestinal microbiota (the good bacteria in our gut) signal to the brain in a myriad of ways. This delicate balance of gut microbes can influence emotional development, modulation of stress and pain, mental health, and neurotransmitter systems in the brain.
Research encourages that improvements in our gut microbiome can improve overall mood, anxiety-like symptoms, pain, and more in people with autism.
There is substantial evidence that using prebiotics and probiotics, such as strains of Lactobacillus, can have a positive effect on the gut-brain axis, but is there something more at play?
In this article I share the results of a cutting edge study that examines the gut brain axis in autism. I give you my analysis, what’s lacking, and key takeaways for those wanting to be informed.
The researchers propose amino acids as a potential treatment.
Today, I take a closer look at the ins and outs of this new study, a review paper entitled, The Gut-Immune-Brain Axis in Autism Spectrum Disorders; A Focus on Amino Acids. I explore its findings and discuss practical implications for therapeutic intervention.
How the Gut-Brain Axis Influences Autism
 I’ve written previously on how dealing with your child’s digestive issues can address both gastrointestinal and neurological symptoms of autism. It’s no secret that children with autism spectrum disorder are significantly more likely to have food allergies than those without it.
I’ve written previously on how dealing with your child’s digestive issues can address both gastrointestinal and neurological symptoms of autism. It’s no secret that children with autism spectrum disorder are significantly more likely to have food allergies than those without it.
However, a startling statistic is that children with autism are seven times more likely to experience frequent diarrhea and colitis than those without a developmental disability.
The link between digestive problems and autism is undeniable, and it’s related to inflammatory bowel issues, acid reflux within the gastrointestinal tract, and possibly more. This is partially due to the inflammation that food allergies and digestive issues can bring on, which can be painful and influence behavior negatively.
This brain-gut link also puts these children at risk of nutritional deficiencies, which can aggravate cognition problems and adversely affect immune responses.
Early life environmental factors can also play a part in the development of autism spectrum disorder. The study cites prenatal and postnatal diet, gut microbiota, and immune system triggers as contributors to the disorder’s prevalence.
Furthermore, it addresses the inflammatory responses that are so common in autism spectrum disorder. It proposes they may be linked to the aforementioned common food allergies, causing increased production of pro-inflammatory cytokines and allergy-associated Th2 cytokines.
This study also suggests that microglia and astroglia (certain brain cells) are deregulated in the brains of people with ASD, altering their immune-like responses. An impaired ability to remove toxins that’s commonly found in individuals with autism can play an important role in a compromised immune system.
A look at the weakened ability to detoxify and a less responsive central nervous system makes it clear: the gut brain axis has a profound effect on autism. While you’re reading, be sure to look into my guide on where environmental toxins are commonly hiding and what to do about them.
These behavioral, digestive, inflammatory, and immunological issues all play a part in the nervous system’s role throughout the body. Patients with ASD have a nervous system battered with these issues, playing a complex role in the disorder. While we know that psychobiotics (gut-supporting supplements that positively influence the gut-brain axis) can be of some help with microbial composition and brain interactions, what about amino acids?
The mTOR Pathway and Amino Acids
Preclinical and mouse model studies have indicated that when mice are allergic to their food intake, their reaction produces many similar symptoms to those seen in individuals with autism and alters processing in the prefrontal cortex.
As mast cells and T cells are unusually activated in the brains of those with ASD, the study suggests that their hyperactivation is linked to symptoms associated with autism. If so, food allergies may be a driving force of the symptoms and behaviors associated with ASD, along with increased intestinal permeability.
So, how can amino acids help these digestive woes? Well, it starts with the mTOR pathway. The activation of the mammalian target of rapamycin (mTOR) is reported frequently in cases of autism.
This activation is also noted in many other health problems, such as insulin resistance and tumor formation, and is increasingly studied for its overarching effects on health, neural communication, brain function, and immune cells. Mutations in mTOR pathway-related genes are widely associated with ASD.
While the first instinct to treat these mutations may be pharmacological, the severe immune system detriments can make mTOR inhibitors an extreme prescription to write. This drug can hamper cell growth, and in other cases, increase unwanted cell signaling.
This study proposes that a safer, nutritionally-based approach using amino acids is possible. For me, this type of research is vital — I believe improving nutrition is hope in action.
Amino acids show a promising effect on the mTOR pathway. They do an excellent job at modulating the function of the proteins that translate both global and specifically selected mRNA for mTOR.
While the research isn’t exactly conclusive, it seems that the amino acid’s transporters enter through the plasma membrane, interact with and activate a multi-protein complex, and activate mTOR at appropriate levels.
A promising trial showed that a developed amino acid diet was able to normalize or reduce mTOR signaling in ASD mice, reducing repetitive behaviors and improving social interactions.
Just as exciting, the potential issues in this dietary change do not have the severe side effects that rapamycin dosages have shown. However, the study notes that proof of principle clinical studies are still needed to see the effects of a diet that specifically adds helpful amino acids.
Can amino acids be used to improve symptoms of autism?
It seems likely. Multiple studies have pointed to reducing inflammation as an effective means of improving the symptoms of autism. This evidence further shows just how impactful the gut microbiome is in autism. Used properly, combinations of amino acids can be helpful in combating not only this inflammation, but harmful mutations as well.
A promising preclinical trial mentioned above developed a combination of relatively higher amounts of histidine (His), lysine (Lys), and threonine (Thr) and relatively lower amounts of leucine (Leu), isoleucine (Ile), and valine (Val) for further studies in ASD, and saw a reduction in mTOR hyperactivity.
Another in vitro study found that dosages of Leu, Ile and Val individually reduced the mRNA and protein levels of the pro-inflammatory cytokine IL-6. It can be inferred from the results of amino acid tests that increasing the availability of amino acids has a positive, anti-inflammatory result on immune cells.
Conversely, some evidence points to a lowered amount of amino acids as a detriment to antibody production. Immunologically speaking, it seems that certain amino acids reduce inflammatory response and assist in antibody production.
In rats with colitis, a condition seen seven times more frequently in those with ASD than neurotypical individuals, amino acid mixtures were seen to positively affect intestinal function and balance gut microbiota composition.
So, what does this mean for nutrition and patients with ASD? Well, with guidance from a doctor or nutritionist, here’s what we may be able to implement: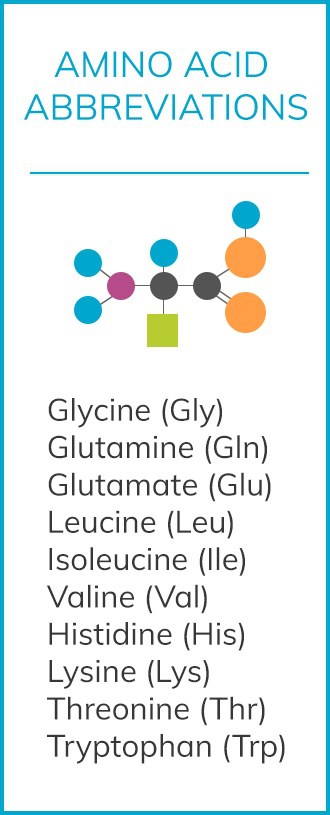
- Raising levels of Gln, Gly, Val, Ile, and Leu can decrease inflammatory response in the immune system.
- Trp and Gly lower intestinal inflammation, while Glu, Gln, and Thr all improve the epithelial barrier, leading to less harmful intestinal permeability.
- Leu, Ile, and Val raise mast cells and epithelial cells in mTOR pathways, while His, Thr, and Lys all lower it while also negatively affecting brain function when used in tandem. These latter three amino acids have a “synergistically negative effect.” Both of these groupings affect the brain in converse ways, which is why it’s important to work on this type of therapy with a licensed doctor or nutritionist/dietician.
- Leu, Ile, and Val also lower pathogenic bacteria levels in the microflora. However, these same three also lower the neuroprotective factor microglia.
- There are many more amino acid combinations, but as we can see, supplementing amino acids must be done mindfully and with proper healthcare supervision. Some can have unintended effects that augment undesirable symptoms in individuals with autism. However, keeping in mind possible negative or synergistic effects, we can begin to formulate the best ratios of potentially helpful amino acid mixtures.
Areas of Further Research and Conclusions
While I find this study to be rather comprehensive in its approach, there is still much more to be done. Firstly, almost all of the current research on amino acid interaction with the gut brain axis and its effect on autism is still in germ-free mice. This is certainly a limitation.
Progression to clinical trials at some point will tell us much more about the possibilities for the future.
I’d also like to hear more from this research about the practical implications for nutritionists and parents seeking to make dietary adjustments. While there are some takeaways, I felt there were limitations in how to apply our current understanding of the link between amino acids, mTOR, inflammation, and brain development.
It is also worth further exploring how to balance each amino acid in combination — some can have extremely neuroinflammatory effects when out of balance. This is the opposite of our goal in helping those with ASD.
Even with its limitations, this study provides us an important look into mTOR in autism and how amino acids may be helpful in improving the gut-brain axis.
In Summary
- The gut brain axis is a significant issue in patients with ASD. Outside of behavioral issues, this is not only a sign but also an aggravation of ongoing symptoms associated with individuals with autism.
- Rectifying an imbalance in gut bacteria can positively affect not only behavior, but also inflammation and digestive symptoms.
- The studied amino acids show promise of modulating negative mTOR pathway signaling, which may reduce a great number of issues that present with ASD.
- If administered properly, neuroactive amino acids show great promise of treating the gut brain axis imbalance in autism, with far fewer side effects than rapamycin medication.
- These findings call for a closer look into amino acids and their properties in relationship to ASD. There are many possible uses for nutritional interventions and alleviation of symptoms within this study.
Sources
-
- Carabotti, M., Scirocco, A., Maselli, M. A., & Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of gastroenterology: quarterly publication of the Hellenic Society of Gastroenterology, 28(2), 203. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4367209/
- Mayer, E. A., Tillisch, K., & Gupta, A. (2015). Gut/brain axis and the microbiota. The Journal of clinical investigation, 125(3), 926-938. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4362231/
- Cryan, J. F., & Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews neuroscience, 13(10), 701. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/22968153
- Mazzoli, R., & Pessione, E. (2016). The neuro-endocrinological role of microbial glutamate and GABA signaling. Frontiers in microbiology, 7, 1934. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5127831/
- Liu, X., Cao, S., & Zhang, X. (2015). Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. Journal of agricultural and food chemistry, 63(36), 7885-7895. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/26306709
- Jyonouchi, H. (2009). Food allergy and autism spectrum disorders: is there a link?. Current allergy and asthma reports, 9(3), 194-201. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/19348719
- Krigsman, A., Boris, M., Goldblatt, A., & Stott, C. (2010). Clinical Presentation and Histologic Findings at Ileocolonoscopy in Children with Autistic Spectrum Disorder and Chronic Gastrointestinal Symptoms. Autism Insights, (2). Full text: http://www.autismone.com/sites/default/files/f_1816-AUI-Clinical-Presentation-and-Histologic-Findings-at-Ileocolonoscopy-in-Ch.pdf_2540%5B1%5D.pdf
- Horvath, K., Papadimitriou, J. C., Rabsztyn, A., Drachenberg, C., & Tildon, J. T. (1999). Gastrointestinal abnormalities in children with autistic disorder. The Journal of pediatrics, 135(5), 559-563. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/10547242
- Schieve, L. A., Gonzalez, V., Boulet, S. L., Visser, S. N., Rice, C. E., Braun, K. V. N., & Boyle, C. A. (2012). Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006–2010. Research in developmental disabilities, 33(2), 467-476. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/22119694
- Horvath, K., & Perman, J. A. (2002). Autistic disorder and gastrointestinal disease. Current opinion in pediatrics, 14(5), 583-587. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/12352252
- Arnold, G. L., Hyman, S. L., Mooney, R. A., & Kirby, R. S. (2003). Plasma amino acids profiles in children with autism: potential risk of nutritional deficiencies. Journal of Autism and Developmental Disorders, 33(4), 449-454. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/12959424
- de Magistris, L., Picardi, A., Siniscalco, D., Riccio, M. P., Sapone, A., Cariello, R., … & Iardino, P. (2013). Antibodies against food antigens in patients with autistic spectrum disorders. BioMed research international, 2013. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3747333/
- Vuong, H. E., & Hsiao, E. Y. (2017). Emerging roles for the gut microbiome in autism spectrum disorder. Biological psychiatry, 81(5), 411-423. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5285286/
- Van Sadelhoff, J., Perez-Pardo, P., Wu, J., Garssen, J., Van Bergenhenegouwen, J., Hogenkamp, A., … & Kraneveld, A. D. (2019). The gut-immune-brain axis in autism spectrum disorders; a focus on amino acids. Frontiers in endocrinology, 10, 247. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/31057483
- Hashim, H., Abdelrahman, H., Mohammed, D., & Karam, R. (2013). Association between plasma levels of transforming growth factor-β1, IL-23 and IL-17 and the severity of autism in Egyptian children. Research in Autism Spectrum Disorders, 7(1), 199-204. Abstract: https://www.sciencedirect.com/science/article/pii/S1750946712001006
- Morgan, J. T., Chana, G., Pardo, C. A., Achim, C., Semendeferi, K., Buckwalter, J., … & Everall, I. P. (2010). Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological psychiatry, 68(4), 368-376. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/20674603
- Alabdali, A., Al-Ayadhi, L., & El-Ansary, A. (2014). A key role for an impaired detoxification mechanism in the etiology and severity of autism spectrum disorders. Behavioral and Brain Functions, 10(1), 14. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/24776096
- Dinan, T. G., Stanton, C., & Cryan, J. F. (2013). Psychobiotics: a novel class of psychotropic. Biological psychiatry, 74(10), 720-726. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/23759244
- De Theije, C. G., Wu, J., Koelink, P. J., Korte-Bouws, G. A., Borre, Y., Kas, M. J., … & Kraneveld, A. D. (2014). Autistic-like behavioural and neurochemical changes in a mouse model of food allergy. Behavioural brain research, 261, 265-274. Abstract: https://www.sciencedirect.com/science/article/pii/S0166432813007511
- Theoharides, T. C., Angelidou, A., Alysandratos, K. D., Zhang, B., Asadi, S., Francis, K., … & Kalogeromitros, D. (2012). Mast cell activation and autism. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1822(1), 34-41. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/21193035
- Hsiao, E. Y., McBride, S. W., Hsien, S., Sharon, G., Hyde, E. R., McCue, T., … & Patterson, P. H. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell, 155(7), 1451-1463. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/24315484
- Laplante, M., & Sabatini, D. M. (2009). mTOR signaling at a glance. Journal of cell science, 122(20), 3589-3594. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2758797/
- Ehninger, D., & Silva, A. J. (2009). Genetics and neuropsychiatric disorders: treatment during adulthood. Nature medicine, 15(8), 849. Abstract: https://www.nature.com/articles/nm0809-849
- Ehninger, D., Han, S., Shilyansky, C., Zhou, Y., Li, W., Kwiatkowski, D. J., … & Silva, A. J. (2008). Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nature medicine, 14(8), 843. Abstract: https://www.nature.com/articles/nm1788
- Fischer, K. E., Gelfond, J. A., Soto, V. Y., Han, C., Someya, S., Richardson, A., & Austad, S. N. (2015). Health effects of long-term rapamycin treatment: the impact on mouse health of enteric rapamycin treatment from four months of age throughout life. PLoS One, 10(5), e0126644. Abstract: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4433347/
- Li, J., Kim, S. G., & Blenis, J. (2014). Rapamycin: one drug, many effects. Cell metabolism, 19(3), 373-379. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3972801/
- Kimball, S. R., & Jefferson, L. S. (2006). New functions for amino acids: effects on gene transcription and translation. The American journal of clinical nutrition, 83(2), 500S-507S. Full text: https://academic.oup.com/ajcn/article/83/2/500S/4650399
- Wu, J., de Theije, C. G., da Silva, S. L., Abbring, S., van der Horst, H., Broersen, L. M., … & Kraneveld, A. D. (2017). Dietary interventions that reduce mTOR activity rescue autistic-like behavioral deficits in mice. Brain, behavior, and immunity, 59, 273-287. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/27640900
- Lee, J. H., Park, E., Jin, H. J., Lee, Y., Choi, S. J., Lee, G. W., … & Paik, H. D. (2017). Anti-inflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food science and biotechnology, 26(5), 1371-1377. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/30263672
- Petro, T. M., & Bhattacharjee, J. K. (1981). Effect of dietary essential amino acid limitations upon the susceptibility to Salmonella typhimurium and the effect upon humoral and cellular immune responses in mice. Infection and immunity, 32(1), 251-259. Abstract: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC350614/
- Liu, X., Beaumont, M., Walker, F., Chaumontet, C., Andriamihaja, M., Matsumoto, H., … & Tomé, D. (2013). Beneficial effects of an amino acid mixture on colonic mucosal healing in rats. Inflammatory bowel diseases, 19(13), 2895-2905. Abstract: https://academic.oup.com/ibdjournal/article-abstract/19/13/2895/4604862?redirectedFrom=fulltext
- Faure, M., Mettraux, C., Moennoz, D., Godin, J. P., Vuichoud, J., Rochat, F., … & Corthésy-Theulaz, I. (2006). Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium–treated rats. The Journal of nutrition, 136(6), 1558-1564. Full text: https://academic.oup.com/jn/article/136/6/1558/4664394
- El-Ansary, A., & Al-Ayadhi, L. (2014). GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. Journal of neuroinflammation, 11(1), 189. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4243332/
- Lee, J. H., Park, E., Jin, H. J., Lee, Y., Choi, S. J., Lee, G. W., … & Paik, H. D. (2017). Anti-inflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food science and biotechnology, 26(5), 1371-1377. Abstract: https://www.ncbi.nlm.nih.gov/pubmed?Db=pubmed&Cmd=ShowDetailView&TermToSearch=30263672
- Ruth, M. R., & Field, C. J. (2013). The immune modifying effects of amino acids on gut-associated lymphoid tissue. Journal of animal science and biotechnology, 4(1), 27. Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3750756/
- Wu, X., Zhang, Y., Liu, Z., Li, T. J., & Yin, Y. L. (2012). Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa morphology and epithelium cell proliferation in weanling piglets. Journal of animal science, 90(suppl_4), 337-339. Abstract: https://www.ncbi.nlm.nih.gov/pubmed/23365372
- Prizant, R. L., & Barash, I. (2008). Negative effects of the amino acids Lys, His, and Thr on S6K1 phosphorylation in mammary epithelial cells. Journal of cellular biochemistry, 105(4), 1038-1047. Abstract: https://www.ncbi.nlm.nih.gov/pubmed?Db=pubmed&Cmd=ShowDetailView&TermToSearch=18767117
- Yang, Z., Huang, S., Zou, D., Dong, D., He, X., Liu, N., … & Huang, L. (2016). Metabolic shifts and structural changes in the gut microbiota upon branched-chain amino acid supplementation in middle-aged mice. Amino acids, 48(12), 2731-2745. Abstract: https://pubag.nal.usda.gov/catalog/5696873




Can you give us the protocol of products to buy so we can put our kids on it. There are so many products and it is hard to pick the correct ones. I like to talk with you and can give you my phone number. Thanks